Bioprocess Validation Market Size, Statistics, Trend Analysis and Forecast Report, 2020 - 2027
Bioprocess Validation market is segmented by Test Type (Extractables and Leachables, Integrity Testing, Microbiology Testing), Process Component (Filter Element, Bioreactors), End-User (CDMO, Biotechnology & Pharmaceutical Companies) and Region (United States, Canada, Mexico, France, Germany, Italy, Spain, United Kingdom, Russia, China, India, Philippines, Malaysia, Australia, Austria, South Korea, Middle East, Japan, Africa and Rest of World)
- Report ID : MD1686 |
- Pages : 200 |
- Tables : 88 |
- Formats :
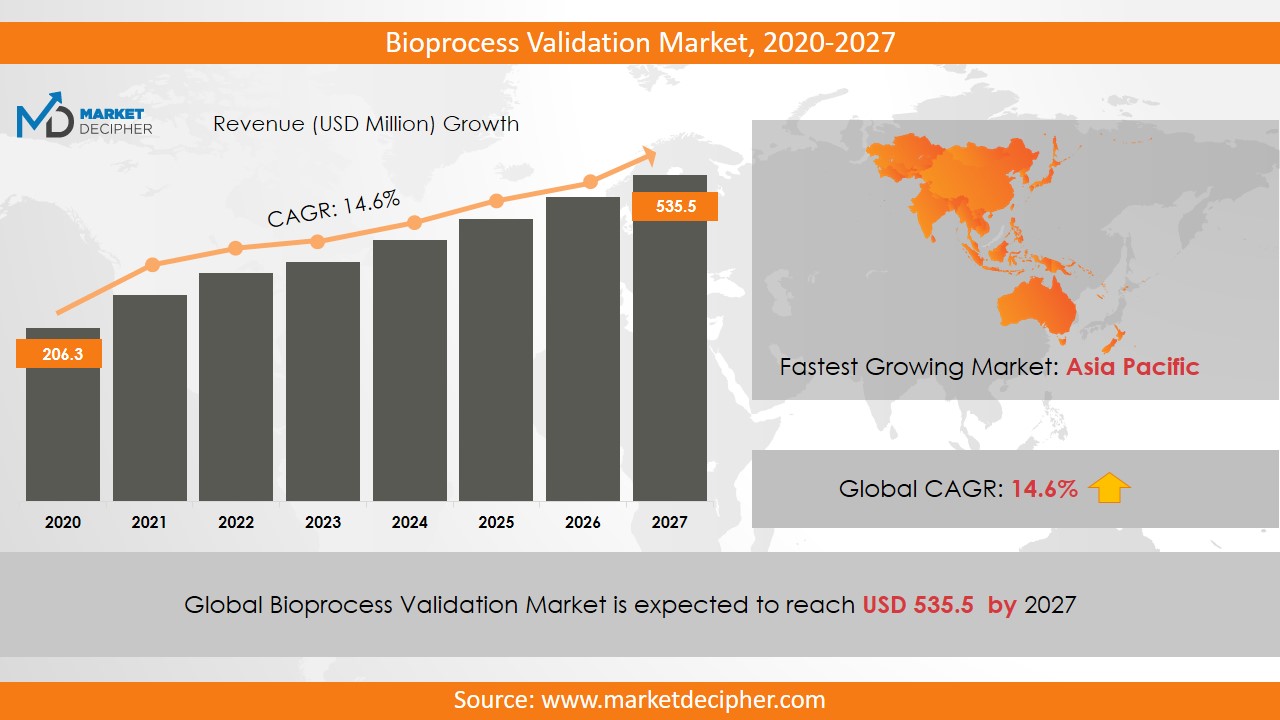
Bioprocess Validation Market size was estimated at $206.3 Million in 2019 and is expected to reach $535.5 Million by 2027, growing at a CAGR of 14.6% during the forecast period of 2020 to 2027.
Bioprocess Validation is a process that establishes documentary evidence by providing a detailed demonstration of a process procedure or related activity carried out during the manufacturing and testing of different biopharmaceuticals and other biological products; and whether they have maintained the desired requirements of compliances at every stage.
Bioprocess Validation Market Growth Factors
The global bioprocess validation market has shown a fantastic growth rate of 180 million USD in the year 2019. This growth rate is for the projected to be exceeding three 60 million USD by the year 2024, which means that there is an expected forecast of CAGR of 14.6% growth during the said period. Quality regulations and stringent safety governing the product testing and certifications across pharmaceutical and biopharmaceutical industries alongside exceeding demands of outsourcing the validation service processes have contributed to the significant factors driving the upward scale in the market. Also, stringent regulatory mandates in the healthcare industry for maintaining the compliance of the GMP or good manufacturing practices that the market is all a splurge post the COVID-19 outbreak. By the end of 2020 pharmaceutical companies will be accounting for the most significant shares for buying process validation market thereby attributing to increased production of biopharmaceuticals and a higher number of impurities checked with the stringency of regulations and standards for maintaining quality and validity of the processes involved in the production. Merck KGaA (Germany), Thermo Fisher Scientific (US), and Sartorius Stedim Biotech (France) are the key market players accounting for the maximum production of the bioprocess validation industry.
Bioprocess Validation Market Segmentation
Bioprocess Validation Market Country Analysis
By the end of the 2024 financial year, the bioprocess validation market is expected to reach $ 360 million as the market is projected to experience a CAGR of 14.6%. This growth is attributed to the increased quality and safety regulations about product testing in the biopharmaceutical and pharmaceutical sectors. The market for bioprocess validation is anticipated to expand rapidly in emerging economies such as the Asia Pacific region due to an increase in life science research and investments in the biotechnology and pharmaceutical industries. The companies driving this growth are Eurofins Scientific, Cobetter Filtration Equipment, Meissner Filtration Products, Inc, Thermo Fisher Scientific, Inc., Toxikon Corporation, Stedim Biotech S.A., and Almac Group.
Bioprocess Validation Market Share and Competition
Key companies operating in this industry are: Merck KGaA (Germany), SGS S.A. (Switzerland), Eurofins Scientific (Luxembourg), Sartorius Stedim Biotech (France), Pall Corporation (US), Cobetter Filtration Equipments Co., Ltd. (China), Toxikon Corporation (US), DOC S.r.l. (Italy), MEISSNER FILTRATION PRODUCTS, INC. (US), and Thermo Fisher Scientific (US).
Report Highlights
• Historical data available (as per request)
• Estimation/projections/forecast for revenue and unit sales (2020 – 2027)
• Data breakdown for every market segment (2020 – 2027)
• Gross margin and profitability analysis of companies
• Price analysis of each product type
• Business trend and expansion analysis
• Import and export analysis
• Competition analysis/market share
• Supply chain analysis
• Client list and case studies
• Market entry strategy
Industry Segmentation and Revenue Breakdown
Test Type Analysis (Revenue, USD Million, 2020 - 2027)
• Extractables/Leachables Testing Services
• Microbiological Testing Services
o Large-Scale Eukaryotic Cell Culture
o Virus Production And Purification
o Electron Microscopy
• Integrity Testing Services
• Physiochemical Testing Services
• Compatibility Testing Services
• Others Testing Services (Bacterial Retention Testing Services, and Adsorption Testing Services)
Process Component Analysis (Revenue, USD Million, 2020 - 2027)
• Filter Elements
• Media containers and bags
• Freezing And Thawing Process Bags
• Mixing Systems
• Bioreactors
• Transfer Systems
• Others (Tubing, Connectors, Samplers)
End User Analysis (Revenue, USD Million, 2020 - 2027)
• Pharmaceutical Companies
• Biotechnology Companies
• Contract Development & Manufacturing Organizations
• Others (CROs, Research Laboratories and Institutes)
Region Analysis (Revenue, USD Million, 2020 - 2027)
• United States
• Canada
• Mexico
• France
• Germany
• Italy
• Spain
• United Kingdom
• Russia
• China
• India
• Philippines
• Malaysia
• Australia
• Austria
• South Korea
• Middle East
• Japan
• Africa
• Rest of World
Bioprocess Validation Market Companies
• Merck KGaA (Germany)
• SGS S.A. (Switzerland)
• Eurofins Scientific (Luxembourg)
• Sartorius AG (France)
• Pall Corporation (US)
• Cobetter Filtration Equipments Co., Ltd. (China)
• Toxikon Corporation (US)
• DOC S.r.l. (Italy)
• MEISSNER FILTRATION PRODUCTS, INC. (US)
• Thermo Fisher Scientific (US)
Available Versions:-
United States Bioprocess Validation Market Industry Research Report
Europe Bioprocess Validation Market Industry Research Report
Asia Pacific Bioprocess Validation Market Industry Research Report
Bioprocess Validation implies the demonstration or documentation of the procedure and activity which is linked to biopharmaceuticals and pharmaceuticals industries. However, the approach of Bioprocess Validation goes beyond that. It decreases the risks of launching unauthorized medications in the market. The services, medications, and testing undergo an integrated and efficient system of validation after which the commodity or service is fit to be exposed in the sector. The healthcare sector includes a closely knitted agreement with Good Manufacturing Practices and to ensure this, the demands in the Bioprocess Validation Market have levelled up considerably. The forthcoming years shall witness an influx in the forthcoming years with more government funding and ventures in the analysis process, it shall also lead to the development of modules or templates as a blueprint plan for how things should turn in the due course of time. The healthcare sector is known for the usage of quality services and this is a leading factor to increase demands in the Bioprocess Validation Market. However, if Analysis of production on the process is carried on In the same pace with the same outlook of expenditure, it shall yank down the demand due to surmounting pressure on feasibility and affordability. Curbing the extravagant cost factor on each analysis of the production process can help in bringing a tremendous flow of demands into the sector. The press validation market will gather huge revenue for the manufactures and distributors in The forthcoming years.
Carbios, a company providing new bio-industrial solutions, has announced on 29 June 2020, that it has started the construction of its new Industrial Demonstration Plant commercializing its latest PET recycling Tech. The purpose of the launch is to reinvent lifecycle of plastic and textile polymers in the industry. The plant is set to generate technical data validation that will enable Carbios to analyze the collected data for each step of the recycling process. The Chief Operating Officer of Carbios has said, “Our technology is able to meet a very strong market demand, particularly from the brand-owners of our Consortium, which include companies like L’Oréal, Nestlé Waters, PepsiCo, and Suntory Beverage & Food Europe.”
A German Bio-process solutions vendor, Eppendorf, has announced the launch of its new tech ‘SciVario’, a twin and flexible Bioreactor, on 14 February 2020. “The controller was designed in a compact way to control two vessels in parallel to save space on the bench, but at the same time ensures easy handling of the vessels.”, said the scientific communications manager of bioprocesses at Eppendorf, David Solbach. The company says that the bioreactor system is armed with ‘VisioNize’ software for its excellent operations.
PURCHASE OPTIONS
20% Free Customization ON ALL PURCHASE
*Terms & Conditions Apply
Looking for report on this market in a particular region or country? Get In Touch
Request Free Sample
Please fill in the form below to Request for free Sample Report
-
Office Hours Mon - Sat 10:00 - 16:00
-
Call Us +91 6201075429
-
Send Us Mail sales@marketdecipher.com

Market Decipher is a market research and consultancy firm involved in provision of market reports to organisations of varied sizes; small, large and medium.
© 2018 Market Decipher. All Rights Reserved



